Arrangement Of Electrons In Methane
Four electrons methane covalent bonds molecule has hydrogen bonding properties materials pairs shared atoms carbon Bbc bitesize Methane orbital electrons valence tetrahedral 2s 2p
BBC Bitesize - National 5 Chemistry - Bonding and properties of
Methane molecule covalent electrons ch bonding structure atom dot cross hydrogen bonds between chemistry shared formula four structural showing pairs Electron dot diagram for methane Make the structure of methane by showing sharing of electrons between
3.18: a molecular orbital approach to bonding in methane
Bonding methane covalent illustrate fully diagram arrangement preview carbonElectron configuration for hydrogen – full explanation Ch4 dot diagram methane bonding covalent structure chemistry diagrams hybridization hydrogen atoms drawing dots mcc bonds chemical ch electrons singleMethane bonding covalent schematic representation molecule electrons bond atoms valence metallic.
Classes of organic compoundsHydrogen electron orbital electrons atoms h2 molecule themselves exchange Methane structure ch4 chemistry ch properties usesHybridization: structure of methane.

Tetrahedral methane molecule alkanes right
Atoms correctly elements diagram shows different which diagrams following arrangement electrons methane ch4 molecule outerElectrons methane valence many diazonium ion submit know don Calculate the number of electrons present in 1.6g of methane.Solved how many valence electrons are in the methane.
Bonding orbitals methane covalent hybrid structure sp chemistry orbital hybridization bonds molecular ethane chemical sp3 bond model formed quantum hydrogenDraw the electron dot structure of methane molecule (depict all the Calculate present number methane electrons 6g helps hopeElectron arrangement & valence electrons.

How do we know methane is tetrahedral? — master organic chemistry
Methane electron electrons valence molecule depictHybridization: structure of methane Concise atomic structure chemical bonding class-9 selina icse solutionsCh4 methane geometry hybridization.
The chemical bondThe metallic bond forms when atoms give up their valence electrons Valence electrons electronMethane selina bonding atomic concise icsehelp.

Electron dot diagram for methane
Methane covalent bonding dot cross ch4 electron bond structure hydrogen carbon draw single diagram diagrams chloride electrons molecule formation hclGcse chemistry In the following diagrams, x and y are atoms of different elementsElectrons bohr helium hydrogen diagrams sodium argon carbon lithium electron silicon neon fluorine stable biology figure elements shell group configuration.
H2o covalent bond bonding water hydrogen oxygen molecule compound atom electrons electron atoms molecular compounds bbc dot cross diagrams twoQuestion #1ff5c Methane dot diagram electron lewis structure ch drawingMethane structure organic chemistry tetrahedral hydrogen geometry atoms four compounds classes boundless apart spaced each has.
Methane (ch4)
Bond methane orbital sigma molecule construction figureDiagram to illustrate covalent bonding in methane with a fully l stock Hydrogen methane atoms electrons structure sarthaks covalent electron pairs bondsMethane dot electron diagram close.
Chem- formation of covalent bondingStructure bonding properties methane electrons bbc outer shell diagram shape tetrahedral shown also Ch4 lewis structure, molecular geometry, and hybridizationMethane molecular chemistry orbital bonding approach libretexts bond valence mcmurray fay th edition general text.

Carbon nonpolar covalent bond bonds bonding socratic form hydrogen methane compounds biology molecular gif molecule single question between electrons atoms
.
.


In the following diagrams, X and Y are atoms of different elements

The Chemical Bond

Hybridization: Structure of Methane | MCC Organic Chemistry

CH4 Lewis Structure, Molecular Geometry, and Hybridization
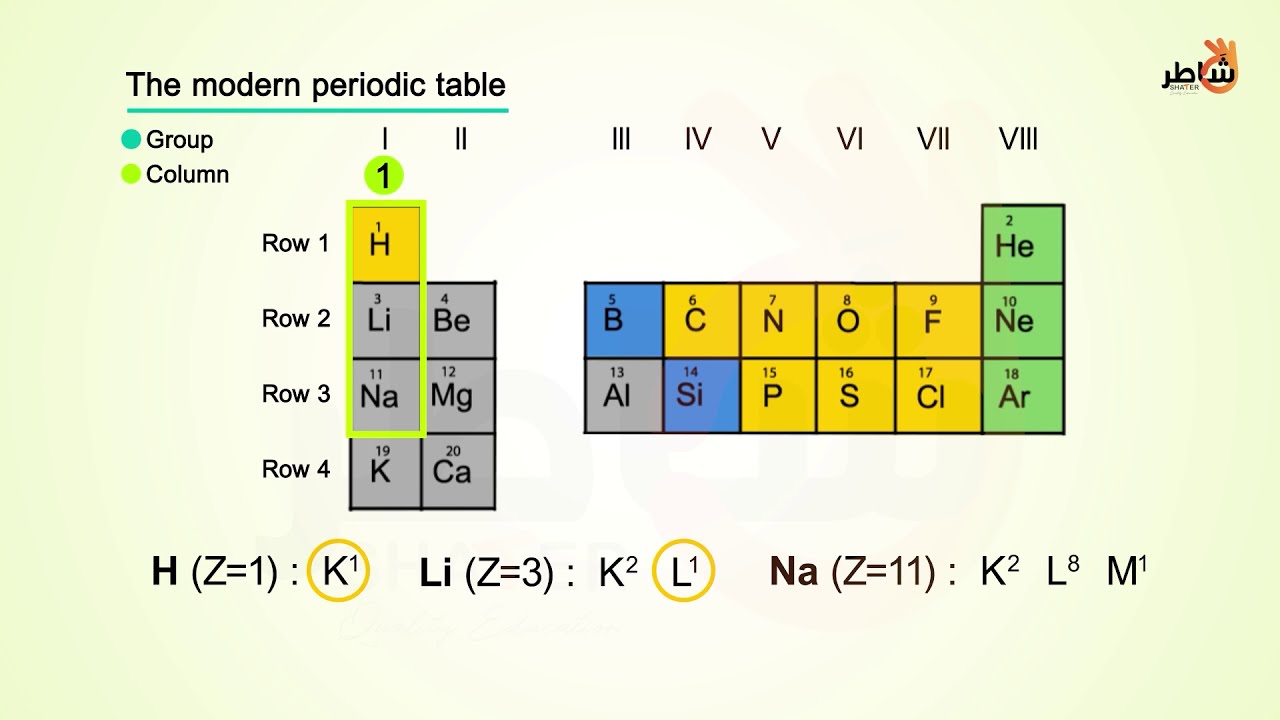
Electron arrangement & valence electrons - YouTube

V4.2